By building up a lab-engineered model of the human blood-brain barrier (BBB), neuroscientists at MIT’s Picower Institute for Learning and Memory have found how the most widely recognized Alzheimer’s ailment chance quality makes amyloid protein plaques disturb the brain’s vasculature and demonstrated they could forestall the harm with meds previously affirmed for human use.
Around 25 percent of individuals have the APOE4 variation of the APOE quality, which puts them at significantly more serious hazard for Alzheimer’s malady. Nearly everybody with Alzheimer’s, and even some older individuals without, experience the ill effects of cerebral amyloid angiopathy (CAA), a condition wherein amyloid protein stores on blood vessel walls disables the capacity of the BBB to appropriately transport nutrients, get out waste and forestall the attack of pathogens and undesirable substances.
In the new examination, distributed June 8 in Nature Medicine, the scientists pinpointed the particular vascular cell type (pericytes) and atomic pathway (calcineurin/NFAT) through which the APOE4 variation advances CAA pathology.
The exploration shows that in individuals with the APOE4 variation, pericytes in their vessels produce an excess of APOE protein, clarified senior creator Li-Huei Tsai, Picower Professor of Neuroscience and executive of the Picower Institute. APOE causes amyloid proteins, which are progressively copious in Alzheimer’s illness, to cluster together.
In the interim, the infected pericytes’ expanded enactment of the calcineurin/NFAT sub-atomic pathway seems to support the raised APOE expression.
There are now tranquilizes that stifle the pathway. As of now they are utilized to stifle the immune system after a transplant. At the point when the specialists directed a portion of those medications, including cyclosporine An and FK506, to the lab-developed BBBs with the APOE4 variation, they collected substantially less amyloid than untreated ones.
“We identify that there is a specific genetic pathway that is expressed differently in a population that is susceptible to Alzheimer’s disease,” said study lead creator Joel Blanchard, a postdoc in Tsai’s lab. “By identifying this we could identify drugs that change this pathway back to a non-diseased state and correct this outcome that’s associated with Alzheimer’s.”
Building barriers
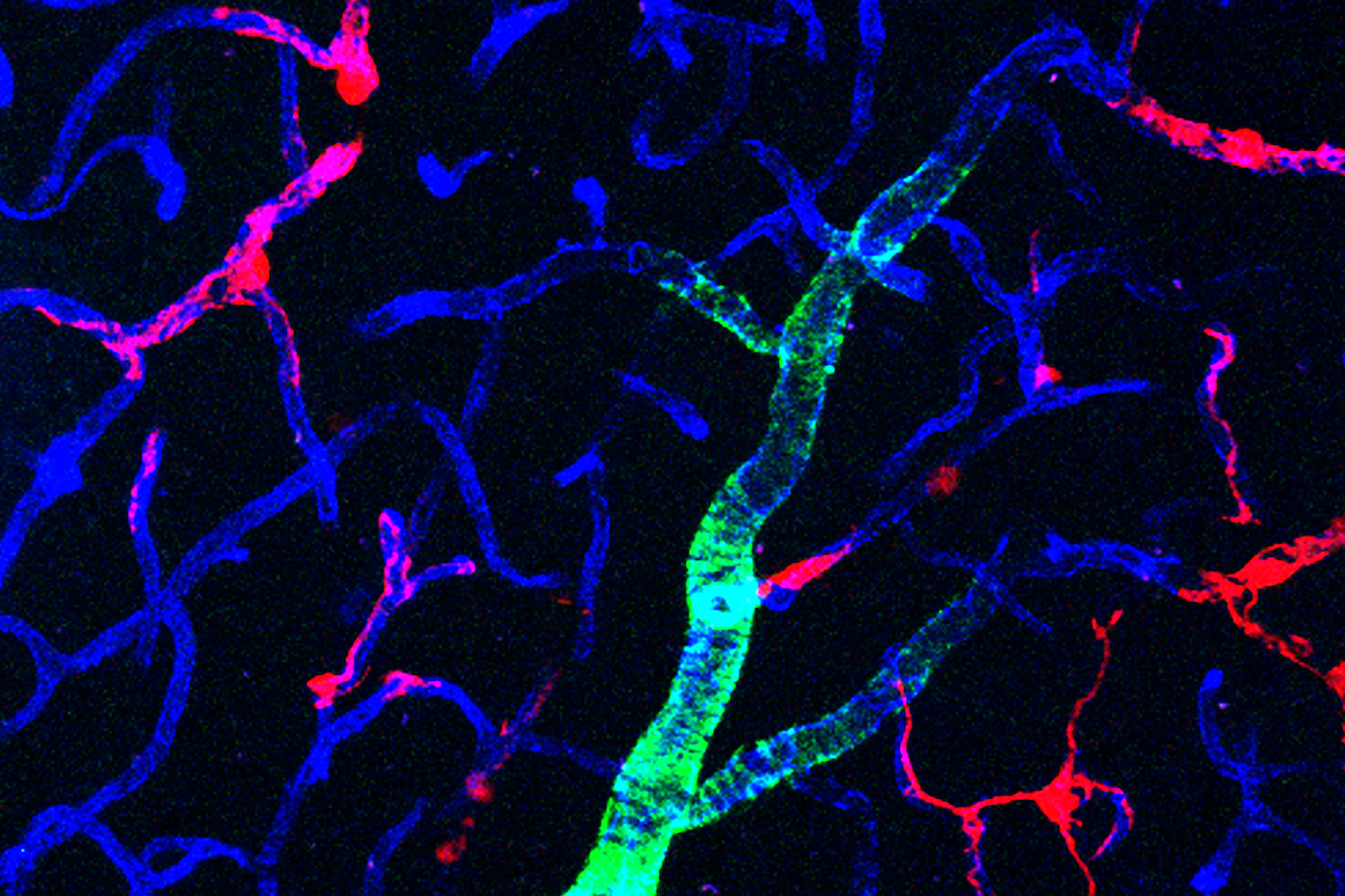
To research the association between Alzheimer’s, the APOE4 variation and CAA, Blanchard, Tsai and co-creators persuaded human actuated pluripotent stem cells to turn into the three kinds of cells that make up the BBB: mind endothelial cells, astrocytes and pericytes. Pericytes were displayed by wall painting cells that they tried broadly to guarantee they showed pericyte-like properties and quality expression.
Developed for about fourteen days inside a three-dimensional hydrogel platform, the BBB model cells amassed into vessels that showed normal BBB properties, including low penetrability to atoms and articulation of a similar key qualities, proteins and sub-atomic siphons as characteristic BBBs.
When drenched in culture media high in amyloid proteins, imitating conditions in Alzheimer’s malady minds, the lab-developed BBB models showed a similar sort of amyloid aggregation found in human ailment.
With a model BBB built up, they at that point tried to test the distinction APOE4 makes. They appeared by a few estimates that APOE4-conveying BBB models aggregated more amyloid from culture media than those conveying APOE3, the more run of the mill and healthy variation.
To pinpoint how APOE4 has that effect, they designed eight distinct variants covering all the potential blends of the three cell types having either APOE3 or APOE4. At the point when uncovered these month-old models to amyloid-rich media, just forms with APOE4 pericyte-like wall painting cells indicated over the top collection of amyloid proteins.
Supplanting APOE4 painting cells with APOE3-conveying ones diminished amyloid affidavit. These outcomes put fault for CAA-like pathology soundly on pericytes.
To additionally approve the clinical significance of these discoveries, the group likewise took a gander at APOE articulation in tests of human cerebrum vasculature in the prefrontal cortex and the hippocampus, two locales significantly influenced in Alzheimer’s infection.
Reliable with the group’s lab BBB model, individuals with APOE4 indicated higher articulation of the quality in the vasculature, and explicitly in pericytes, than individuals with APOE3.
“That is a salient point of this paper,” said Tsai, an establishing individual from MIT’s Aging Brain Initiative. “It’s really cool because it stresses the cell-type specific function of APOE.”
A pathway toward treatment?
The following stage was to decide how APOE4 turns out to be so overexpressed by pericytes. The group in this way distinguished many interpretation factors – proteins that decide how qualities are communicated – that were controlled distinctively somewhere in the range of APOE3 and APOE4 pericyte-like wall painting cells.
At that point they scoured that rundown to see which factors explicitly sway APOE articulation. A lot of elements that were upregulated in APOE4 cells stuck out: ones that were a piece of the calcineurin/NFAT pathway. They watched comparative upregulation of the pathway in pericytes from human hippocampus tests.
As a feature of their examination of whether raised flagging movement of this pathway caused expanded amyloid statement and CAA, they tried cyclosporine An and FK506 on the grounds that they pack pathway action down.
They found that the medications diminished APOE articulation in their pericyte-like painting cells and in this way APOE4-intervened amyloid stores in the BBB models. They additionally tried the medications in APOE4-conveying mice and saw that the prescriptions decreased APOE articulation and amyloid development.
Blanchard and Tsai noticed that the medications can have huge symptoms, so their discoveries probably won’t propose utilizing precisely those medications to address CAA in patients.
“Instead it points toward the value of understanding the mechanism,” Blanchard said. “It allows one to design a small molecule screen to find more potent drugs that have less off-target effects.”